Martin, Glenroy, Narvaez, Javier, Bulmer, Rachel and Durrant, Marcus (2016) Biotransformation and molecular docking studies of aromatase inhibitors. Steroids, 113. pp. 95-102. ISSN 0039-128X
|
Text
2015 Martin-Correction_MCD.docx - Accepted Version Available under License Creative Commons Attribution Non-commercial No Derivatives 4.0. Download (6MB) |
||
![[img]](/27445/1.hassmallThumbnailVersion/martin%20et%20al%20-%20aromatase%20inhibitors%20-%20graphical%20abstract.jpg)
|
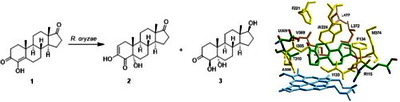
Image (Graphical abstract)
martin et al - aromatase inhibitors - graphical abstract.jpg - Cover Image Download (20kB) | Preview |
Abstract
Bioconversion of the aromatase inhibitor formestane (4-hydroxyandrost-4-ene-3,17-dione) by the fungus Rhizopus oryzae ATCC 11145 resulted in a new minor metabolite 3,5α-dihydroxyandrost-2-ene-4,17-dione and the known 4β,5α-dihydroxyandrostane-4,17-dione as the major product. The structural elucidation and bioactivities of these metabolites are reported herein. Molecular modeling studies of the interactions between these metabolites and the aromatase protein indicated that acidic (D309), basic (R115), polar (T310), aromatic (F134, F221, and W224), and non-polar (I133, I305, A306, V369, V370, L372, V373, M374, and L477) amino acid residues contribute important interactions with the steroidal substrates. These combined experimental and theoretical studies provide fresh insights for the further development of more potent aromatase inhibitors.
| Item Type: | Article |
|---|---|
| Additional Information: | Funding information: This work was partly funded by a grant provided by the National High Magnetic Field Laboratory (NHMFL) user grant (ML-Martin-002), the American Chemical Society (ACS) Project SEED endowment, the Hillsborough County Public Schools Academic Programs, and the ACS Tampa Bay local section. We thank Professor John Pezzuto, Eun-Jung Park and Tamara Kondratyuk (University of Hawaii at Hilo) for conducting aromatase and cytotoxic assays. We are grateful to James Rocca (NHFML) for conducting 1D and 2D NMR experiments. |
| Uncontrolled Keywords: | Aromatase inhibitor; Molecular docking; Rhizopus oryzae; Formestane; Biotransformation; Cytochrome P450 monooxygenase |
| Subjects: | C700 Molecular Biology, Biophysics and Biochemistry |
| Department: | Faculties > Health and Life Sciences > Applied Sciences |
| Depositing User: | Becky Skoyles |
| Date Deposited: | 08 Aug 2016 10:44 |
| Last Modified: | 01 Sep 2022 13:36 |
| URI: | https://nrl.northumbria.ac.uk/id/eprint/27445 |
Downloads
Downloads per month over past year